1s2 2s2 2p6 3s2 3p6 4s2 In writing the electron configuration for neon the first two electrons will go in the 1s orbital.
 La tabla periódica, capas de electrones y orbitales
La tabla periódica, capas de electrones y orbitales
So,this two extra electrons will be attached the.
Electron configuration of neon ion. The electron configuration of a fluoride ion, f⁻, is _____ a. Electronic configurations describe each electron as moving independently in an orbital, in an average field created by all other orbitals. Therefore, br has 1 unpaired electron.
To use electron affinities properly, it is essential to keep track of sign. Note that when writing the electron configuration for an atom like fe, the 3d is usually written before the 4s. The same principles can be applied to many other cases.
Neon has the electron configuration ne2,8. Neon is a chemical element with the symbol ne and atomic number 10. In atomic physics and quantum chemistry, the electron configuration is the distribution of electrons of an atom or molecule in atomic or molecular orbitals.
The remaining six electrons will go in the 2p orbital. However, notice that 1s 2 2s 2 2p 6 3s 2 3p 6 is the configuration for argon, a noble gas. We rewrite the electron configuration:
When an electron is added to a neutral atom, energy is released. Electron configuration chart for all elements in the periodic table. Zinc's full electron configuration is:
The electronic configuration of an atom gives us the idea of how the electrons of the atom are distributed in different orbits and orbitals in the increasing order of the energy. Therefore, you should write the electron configuration for 18 electrons. The br atom has 4s 2 3d 10 4p 5 as the electron configuration.
The electron configuration is the same as for neon and the number of nonvalence electrons is 2. Thus, you should write the electron configuration for 10 electrons. Since 1s can only hold two electrons the next 2 electrons for ne go in the 2s orbital.
Aluminum has the electron configuration [ne]3s23p1. For example, consider the compound formed from aluminum and oxygen. 1s2 2s2 2p6 3s2 3p6 4s1:
Neon is the tenth element with a total of 10 electrons. For example, the electron configuration of the neon atom is 1s2 2s2 2p6, using the notation explained below. Because it has no unpaired electrons, it is.
The electronic configuration of oxygen is 1s^2 2s^2 2p^4 the charge of oxide ion is —2, which means an oxygen atom gained 2 extra electrons. The b atom has 2s 2 2p 1 as the electron configuration. The atomic number of oxygen is 8.
Neon is in the second period and the group 18 of the periodic table. However, there can be a few positive or negative charge ions with the same number of electrons/electron configuration as neon. Mathematically, configurations are described by slater determinants or configuration state func
Oxygen in a neutral state would have 8 total electrons (6 valence). 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10. Since 1s can only hold two electrons the next 2 electrons for ne go in the 2s orbital.
With 10 electrons you should note that oxygen's electron configuration is now exactly the same as neon's. Once again, the electron configuration is the same as in the previous examples and the number of. What are the six ions that have the same electron configuration as ne?
1s2 2s2 2p6 3s2 3p5: This would add 2 electrons to its normal configuration making the new configuration: We can abbreviate the electron configuration by indicating the innermost electrons with the symbol of the preceding noble gas.
Each element has a unique atomic structure that is influenced by its electronic configuration, which is the distribution of electrons across different orbitals of an atom. In other words 2 electrons in the inner shell and 8 in the outer full shell. To achieve the neon configuration, aluminum must lose three electrons, forming the al3+ ion.
Then move two elements to the left. There are 118 elements in the periodic table. In writing the electron configuration for neon the first two electrons will go in the 1s orbital.
The remaining six electrons will go in the 2p orbital. 1s2 2s2 2p6 3s2 3p1: An atom of neon in the gas phase, for example, gives off energy when it gains an electron to form an ion of neon.
Elemental fluorine has an electron configuration of 1s22s22p5 and needs 1 more electron to complete its 2p orbital which it will acquire in formation of the fluoride ion. For example, the electron configuration of the neon atom (ne) is 1s 2 2s 2 2p 6. The preceding noble gas with an atomic number less than sodium is neon, ne.
In its simplest form, we could write the electronic configuration of chlorine as 2,8,7 in terms of subshells, the electronic configuration would be represented as 1s 2 2s 2 2p 6 3s 2 3p 5. The same as that of a neon atom Therefore, the abbreviated electron configuration of sodium is [ne]3s 1 (the electron configuration of neon is 1s 2 2s 2 2p 6, which can be abbreviated to [he]2s 2 2p 6).
Neon is the tenth element with a total of 10 electrons. 1s 2 2s 2 2p 6 given : 1s2 2s2 2p6 3s2 3p6:
Atoms and atomic structure chemistry noble gases elements and compounds science experiments. According to the laws of quantum mechanics, a certain energy is associated with each. Both of the configurations have the correct numbers of electrons in each orbital, it is just a matter of how the electronic configuration notation.
1s2 2s2 2p6 3s2 3p4: Therefore the ne electron configuration will be 1s 2 2s 2 2p 6. Thus it gains an electron when forming the fluoride ion, and becomes isoelectronic to neon.
The same for na+ which has lost an electron and also is na+2,8. Look at a perodic table and find neon (it's on the far right, second row). Therefore the iron electron configuration will be 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 6.
If you add two electrons to oxygen, it will have 10 electrons (8 valence electrons) which is exactly what neon has. Just replace this portion of zinc's electron notation with argon's chemical symbol in brackets ([ar].) so, zinc's electron configuration written in shorthand is [ar]4s 2 3d 10. In atomic physics and quantum chemistry, the electron configuration is the distribution of electrons of an atom or molecule in atomic or molecular orbitals.
It is a noble gas. Determining the valency of an element. Because it has one unpaired electron, it is paramagnetic.
Therefore the ne electron configuration will be 1s22s22p6. Electron configurations are useful for: Recall, the electron configuration for na is:
 Interactive Periodic Table of the Elements (With images
Interactive Periodic Table of the Elements (With images
 Color Printable Periodic Table Neon Theme Periodic
Color Printable Periodic Table Neon Theme Periodic
 Pop up in LA (With images) Electron configuration
Pop up in LA (With images) Electron configuration
 prepitude Neon signs, Electron configuration, Finding
prepitude Neon signs, Electron configuration, Finding
Periodic Table of Electron Configuration Orgo Pinterest
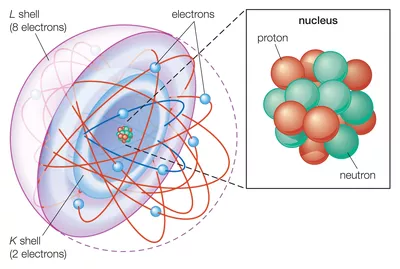 The History of Atomic Theory Led to Quantum Mechanics in
The History of Atomic Theory Led to Quantum Mechanics in
0 Response to "17+ Electron Configuration Of Neon Ion"
Posting Komentar